4D Workshop 2023
WHGA/001
PSI
Aim of the 4D workshop:
The 4D Treatment Planning Workshop for Particle Therapy is dedicated to the treatment of moving targets with scanned particle beams, started in 2009 and since then has been organised annually.
The workshop's mission is to create an informal ground for clinical medical physicists, medical physics researchers and medical doctors interested in the development of the 4D technology, protocols and their translation into clinical practice.
The upcoming workshop is the 14th edition, bringing the 4D workshop back to the PSI after 10 years.
Please note that the registration will close on August 13th, 2023
The Workshop is supported by the Swiss National Science Foundation under grant IZSEZ0_221470 
-
-
08:25
→
08:45
Bus Brugg dep 08:35 -> PSI arr 08:50 20m
-
08:45
→
16:30
4D Workshop
-
08:45
Arrival and registration 30m
-
09:15
Welcome 15m
-
09:30
4D clinical status and challenges: Clinic 40mSpeaker: Prof. Dirk de Ruysscher (Maastricht University)
-
10:10
4D clinical status and challenges: Physics 40mSpeaker: Prof. Christoph Bert (Uniklinikum Erlangen)
-
10:50
Coffee break 25m
-
11:15
Practical session: Case study 1h 15mSpeaker: Dr Katarzyna Czerska (Paul Scherrer Institut)
-
12:30
Lunch 1h 15m
-
13:45
4D outlook/forecast: 4D PT treatment 2013 - 2023 - 2033 45mSpeaker: Antony John Lomax (PSI - Paul Scherrer Institut)
-
14:30
Advanced 4D technology: 4D TPS 40mSpeaker: Prof. Marco Schwarz (University of Washington)
-
15:10
Advanced 4D technology: 4D delivery 40mSpeaker: Prof. Christian Graeff (GSI)
-
15:50
Advanced 4D technology: 4D QA 40mSpeaker: Dr Gabriel Guterres Marmitt (Universitair Medisch Centrum Groningen)
-
08:45
-
16:30
→
19:00
Apéro / Facility Tour / Poster Session
-
16:30
3D-printed materials for end-to-end test phantoms in particle therapy 2h 30mSpeaker: Jacob Brunner
-
16:30
4D MRI and motion management in particle therapy 2h 30mSpeaker: Anestis Nakas
-
16:30
A national phase II study of proton therapy for hepatocellular carcinoma 2h 30m
Introduction
Radiotherapy is an effective local treatment option in patients with hepatocellular carcinoma (HCC). However, photon radiotherapy is not a widely used modality in HCC as most patients have underlying cirrhosis making the liver more sensitive to radiation induced liver disease (RILD).The aim of this national phase II study (NCT05203120) is to investigate feasibility and safety of exhale gated proton therapy in HCC patients with large tumors up to 12 cm and impaired liver function.
Methods
50 patients will be included over 3 years starting from April 2022. Patients must not be candidate for any other curative treatment strategies as resection or radiofrequency ablation (RFA). Maximum 3 tumors in the liver are allowed with a maximum tumor size of 12 cm in total. The maximum Child-Pugh score allowed is B8. Patients are followed closely during and after treatment in order to capture toxicity.Before treatment planning, 2-4 fiducials markers are implanted around the tumor(s). The patients are positioned in supine position with arms up with an external marker box for gating. A 4DCT scan without contrast, a triple phase 3DCT with contrast and an MRI with contrast are obtained for delineations and treatment planning. A GTV is defined using the triple phase 3DCT and a 5 mm isotropic margin is added to create the CTV. A target iCTV is defined on the exhale phase of the 10-phase p4DCT as the union of the CTVs in the 5 phases closest to exhale.
Treatment plans are created on the exhale phase using 3 fields and robust single field optimization
Patients are treated with either 58 GyRBE (if CTV < 1.5 cm to porta hepatis) or 67 GyRBE in 15 fractions.
Daily setup is based on free-breathing CBCT with manual match to the motion-blurred exhale position of the implanted markers. Weekly control CT scans are acquired.
Investigations within the study includes comparative photon VMAT plans, target motion variability between planning 4DCT and respiratory gated treatment fractions and motion-including tumor dose reconstruction for individual treatment fractions.
Results
So far, 18 patients have been treated successfully using gated proton therapy treatment in the study. No replans have been performed based on weekly control CT scans. The mean dose to the healthy liver has been reduced with 39% in average (range, 23% to 60%) compared to comparative photon VMAT plans. Toxicity data have not been analyzed yet.
For the first 6 patients, the full motion of the markers in CC direction was 11.5±3.2mm (mean±SD) during 4DCT and 20.2±4.1mm during CBCT. The 50th percentile motion in CC direction was 3.7±1.4mm (mean±SD) during 4DCT and 4.8±1.7mm during CBCT. Respiratory exhale gating effectively limited both motion and motion variability.Speaker: Britta WEBER (Danish Center for particle Therapy) -
16:30
AI-assisted proton radiography interpretation for fast detection and classification of treatment deviations’ 2h 30mSpeaker: Giuliano Perotti Bernardini
-
16:30
Comparison of two techniques for 4D-robust adaptive proton therapy 2h 30mSpeaker: Suryakant Kaushik
-
16:30
DiffuseRT: Learning Likely Anatomical Deformations From Data 2h 30mSpeaker: Luciano Rivetti
-
16:30
Dose reconstruction strategies using prompt-gamma radiation in proton therapy 2h 30m
INTRODUCTION
To monitor range uncertainties in proton therapy, dose reconstruction is desirable,
and it has already been proposed in the literature starting from detected secondary radiation, mostly positron emitters (PE). Prompt-gammas (PG) can be alternatively used:
emitted within nanoseconds after nuclear interactions, they can be exploited for realtime adaptation.MATERIAL AND METHODS
The purpose of this work is to assess the feasibility of three existing approaches for
dose reconstruction from PG: the analytical deconvolution method [1], the evolutionary
algorithm [2][3][4], and the maximum-likelihood expectation-maximization (MLEM)
algorithm [5][6]. These were chosen because of the inherent application of the filtering,
introduced first by Parodi and Bortfeld [7] for PET data, then extended to PG radiation
by Schumann et al. [2] and by Pinto et al. [8]. Mono- and polyenergetic proton beams irradiation of homogeneous and inhomogeneous phantoms were simulated (using Geant4
[9], v.10.07.p03). 1D laterally integrated depth-dose and depth-PG distributions were
considered.RESULTS
The accuracy of the reconstructed depth-dose profiles was evaluated via comparison
with their corresponding ground truth using different metrics, which show an overall
match. Regardless of the method, ∆R80, ∆R50 and ∆R10 metrics (see table 1 caption)
were found to be below 1 mm. For the tests performed, the methods are sensitive to
properly reconstruct dose when range shifts of different magnitudes are introduced.CONCLUSIONS
This work verifies the applicability of the investigated dose reconstruction techniques to PG distributions. Extension to 3D distributions is currently in progress, and
the application to measured data is foreseen in the near future. The final purpose consists
of the comparison of the evaluated strategies as part of their applicability to real-time
adaptive particle therapySpeaker: Beatrice Foglia -
16:30
Dosimetric Benefit Of Online Daily Adaptive Proton Therapy For Head And Neck Cancer Patients 2h 30mSpeaker: Evangelia Choulilitsa
-
16:30
Fast Proton Treatment Plan Re-Optimization Using The Reference Point Method To Account For Interfractional Anatomical Changes 2h 30mSpeaker: Zihang Qiu
-
16:30
Heterogeneously hypofractionated proton therapy for Locally Advanced non-small cell lung cancer (NSCLC) - HERAN2 trial 2h 30m
The aim of the Danish randomized HERAN2 trial is to examine if heterogeneous and hypofractionated proton radiotherapy can improve survival compared with X-ray radiotherapy in patients with locally advanced NSCLC who are not candidates for standard chemoradiotherapy. Patients selected for proton therapy are treated at the Danish Center for Particle Therapy and hereby, we present our treatment strategy and experience from the first pilot patients in the protocol.
The patients are positioned in standard fixation with arms up when possible, and for all patients, a 4DCT scan without contrast, a 3DCT with contrast and a PET-CT scan are obtained for treatment planning. Delineation and treatment planning is performed on the average scan. Respiratory motion is included by delineation of the gross tumour volume (GTV) on all phases of the 4DCT with a subsequent rigid accumulation into an iGTV on the average phase. The iCTV is hereafter defined by a 5 mm isotropic margin to the iGTV.
The gross tumour volumes of primary tumours (GTV-T) and lymph nodes with a diameter >3cm are prescribed a mean dose of up to 66Gy in 24 fractions, 5 fractions per week while the remaining targets (GTV-N and CTV) are covered by 95% of 50 Gy. The dose escalation is limited by constraints to the surrounding organs at risk (OAR).Treatment plans are created in the Varian Eclipse treatment planning system v16.1 using robust multi-field optimization and the Monte Carlo dose calculation algorithm, AcurosPT. The plans consist of 3-6 fields and robust optimization is performed using 14 scenarios; 4.5% range uncertainty combined with 5 mm and 0 mm setup uncertainty along the major axis. Respiration (excluding interplay effect) is accounted for by coverage of the iCTV and 4D robust evaluation on all the respiratory phases. A workflow for evaluation of the interplay effect is currently being developed for the pilot patients, and will be used to determine if repainting is necessary.
Patients are setup with daily CBCT image guidance using a soft tissue match on the target volume. A thorough adaptive strategy based on daily imaging and weekly surveillance scans is mandatory for all patients, and re-planning is performed if dosimetric or geometric changes exceeds the clinic-specific tolerances.
Speaker: Hanna Rahbek Mortensen (Danish Center for Particle Therapy) -
16:30
Impact of temporal image resolution on the interplay effect in 4D Monte Carlo dose calculation for IMPT 2h 30m
The interplay between the beam delivery time structure and the patient motion makes 4D dose calculation (4DDC) important when treating moving tumors with IMPT. In a conventional 4DDC, each spot is assigned to its nearest phase image in a 4DCT, based on a delivery log and a breathing signal. The doses computed on the phase images are then deformed to a reference phase and accumulated. An apparent source of error in 4DDC is the approximation applied in the nearest-neighbor assignment of spots to phases, due to the coarseness of the temporal image resolution.
We addressed this limitation by applying registration-based interpolation between phase images for increased temporal resolution of the 4DCT. Assuming linear motion between the phases, we deformed each phase image to its neighbor using the deformation vector field that aligns them, scaled by the desired time step. We then applied Monte Carlo-based 4DDC to both the original 4DCT (10 phases), and extended 4DCTs at increasingly fine temporal resolutions (20 - 100 phases).
The method was evaluated on seven NSCLC patients treated with three robustly optimized IMPT beams with spot delivery times of approximately 3 ms and energy switching times of 2 s. Dose differences were compared by computing the volume of the CTV differing by more than 1, 2, and 3 percent of the prescribed dose. The ground truth was taken as the dose computed using 100 phase images, and was justified by considering the diminishing effects of adding more images. For all patients, 20-30 phase images were sufficient to mitigate the errors using 2 and 3 percent thresholds. For three patients, 1 percent errors persisted even at higher resolutions, which was attributed to artefacts and poor image inconsistencies. For the four patients without such problems, 1 percent errors vanished at resolutions beyond 50 images.
To conclude, the inclusion of breathing phases in between the original phases in a 4DCT can remove the approximation errors which arise from the nearest-neighbor assignment in 4DDC.
Speaker: Ivar Bengtsson (RaySearch Laboratories & KTH, Royal Institute of Technology) -
16:30
Integration of anatomical variations into robust plan evaluation for proton therapy 2h 30mSpeaker: Nadine Vatterodt
-
16:30
Inter- and intrafractional 4D dose accumulation for evaluating ΔNTCP robustness in lung cancer 2h 30mSpeaker: Andreas Smolders
-
16:30
Investigation of uncertainty maps to assess deep-learning based synthetic CT for adaptive proton therapy 2h 30mSpeaker: Art Galapon
-
16:30
Joint Translation And Registration Framework: A Novel Approach To Address Misalignment In MR-Based CT Synthesis 2h 30mSpeaker: Xia Li
-
16:30
Online tumor motion forecasting using Vision Transformers 2h 30m
In radiotherapy, forecasting the motion of a lung tumor is a fundamental task for dose delivery. Indeed, anticipating the motion saves time in the decision-making process for real-time applications and could lead to a better homogeneous dose delivery and more healthy tissues spared. Our method is trained and tested on fluoroscopic sequences generated from 4D-Computed Tomography (4DCT) scans which were acquired one week apart. Due to anatomical changes that can happen during the course of the treatment, training and testing on a different 4DCT is challenging. In that way, we simulate the inter fraction variability that can happen during a treatment over several weeks.
Our method consists of training a Vision Transformer network on fluoroscopy images of the first week of the treatment. Those are cropped around the tumor. Then, the network performance is evaluated on the second week of the treatment. Data augmentation using the software OpenTPS is done and the impact of the number of inter/intra-fractions needed is studied.
This study is realised on fourteen patients with various breathing amplitude (up to 5mm in sagittal, 3mm in axial and 11mm in coronal directions) and different tumor sizes and treatment responses. It yields to an average prediction error of 1.30 mm in the three directions at an horizon of 1s. As future steps, we plan to evaluate the robustness of the Vision Transformer at three to four different timepoints and on breathing amplitude up to 40 mm.
Key-words: Vision Transformer, transformers network, 4DCT, fluoroscopy imaging, radiotherapy, respiratory motionSpeaker: Gauthier Rotsart de Hertaing (UCLouvain) -
16:30
Performance of kV-CBCT imaging during megavoltage beam irradiation under phase-gated condition 2h 30m
[Purpose] kV-CBCT imaging during MV beam irradiation technique has been developed on C-arm linac. The purpose of this study was to quantify the performance of the intra-irradiation imaging technique under a phase-gated condition.
[Method] TrueBeam Developer mode was used to perform the intra-irradiation imaging. Catphan504 phantom was used. To demonstrate respiratory movement, the phantom was placed on a moving platform. The phantom was moved along the gun-target direction with the sinusoidal wave of amplitude of 40 mm, and 15 BPM. The intra-irradiation imagings during MV beam delivery were performed. First, intra-irradiation projections were acquired under a gating window of 50% (30-70% phase) with various MV beam energies: 6 MV, 10 MV, 15 MV, 6 MVFFF, and 10 MVFFF. One half- (181 – 21 deg clockwise, small FOV) or one full-rotation (181-179 deg clockwise, large FOV) beams with dose rate and field size of 400 MU/min and 15×15 cm2 were used per MV beam energy. kV imaging parameters were 125 kVp and 600/1080 mAs (small/large FOV). Second, MV-scatter-only projections were acquired with kV collimators closed to correct the MV-scatter signal in the intra-irradiation projections. Third, the acquired intra-irradiation projections were corrected by subtracting the MV-scatter-only projections. Gated and static intra-irradiation CBCT images were reconstructed from corresponding MV-scatter-corrected intra-irradiation projections. The FDK algorithm was used for the image reconstruction. In addition, Gated and static standard CBCT images were reconstructed from standard kV projections with the FDK algorithm. Root-mean-square error (RMSEs) of CT-numbers of inserted rods in the phantom on each intra-irradiation CBCT image were calculated using the CT-numbers on the standard CBCT image as reference. Noise was also calculated as the standard deviation of CT-numbers on the uniform module in the phantom.
[Result] RMSEs of intra-irradiation CBCT images under static condition were approximately less than 30 HU; however, those under gated condition were relatively large (50-230 HU). Larger FOV showed larger RMSEs. RMSEs of the intra-irradiation CBCT images acquired during 6XFFF beam delivery were large compared to the images acquired during other MV beam energies. Noises of intra-irradiation CBCT images under static condition (13-17/21-23 HU for small/large FOV, respectively) were comparable to those of standard CBCT images (12.3/20.8 HU). Noises of intra-irradiation CBCT images under gated condition were 15-23/23-35 HU while those of standard CBCT images were 8.8/27.2 HU. For small FOV, noises of intra-irradiation CBCT images were larger.
[Conclusion] Performance of the intra-irradiation imaging technique during a phase-gated condition was quantified.Speaker: Hiraku Iramina (Department of Radiation Oncology and Image-applied Therapy, Graduate School of Medicine, Kyoto University) -
16:30
Real-time RBE-weighted 4D-dose calculation for carbon ion therapy 2h 30m
Introduction
The estimation of dose errors is a key part of online adaptive particle therapy. Interplay distorts dose distributions leading to non-ideal tumor coverage and higher dose to healthy tissues.
Fast forward dose calculation (FDC) is mandatory to verify the efficacy of motion mitigation strategies during treatment. We propose and validate in silico a system to reconstruct the planned and delivered RBE-weighted 4D-dose in real-time, i.e. within each accelerator cycle (<5 s) of the carbon ion therapy delivery.
Material and Methods
The GPU-based FDC algorithm was based on the existing RIDOS dose calculation tool.
For real-time delivered dose calculation, the fast FDC was interfaced with the research version of the CNAO Dose Delivery System (DDS) through a TCP/IP connection, providing measured and planned spot properties (MU, beam position and motion phase) during the delivery. The algorithm evaluates the cumulative delivered and prescribed dose distributions in parallel to delivery and performs a gamma-analysis comparison at the end of each slice. The results are updated on a graphical user interface (Fig. 1).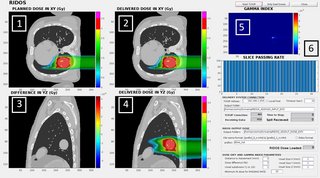
The system has been tested in silico on a virtual XCAT 4DCT, with a simulated data stream from the DDS for dose reconstruction. For verification of advanced motion mitigation strategies, we designed RIDOS to be compatible with the recently developed MultiPhase 4D delivery (MP4D).
Results
The framework successfully performed the FDC of each simulated spill before the end of the accelerator cycle. The overall computation time per spot was ~0.45 ms. The reconstructed dose was validated against the research treatment planning system TRiP (Fig. 2), showing good agreement with gamma-index passing rate (3mm/3%)>98%.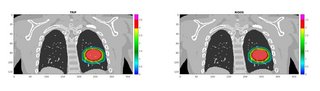
Conclusions
We demonstrated the feasibility of a real-time dose calculation with clinically acceptable precision.
The next step will involve experimental tests with carbon and proton beams at the CNAO facility, as well as consideration of more patient data, with variable motion and motion mitigation strategy. In the future, this framework will open the door for possible real-time adaptive particle therapy.Funded by European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska-Curie grant agreement No 955956
Speaker: Cosimo Galeone -
16:30
Spot-scanning proton therapy for breast cancer in free breathing versus deep inspiration breath-hold 2h 30m
Background
Proton therapy for breast cancer is usually given in free breathing (FB). With the use of deep inspiration breath-hold (DIBH) technique, the location of the heart is shifted more caudally, away from the internal mammary nodes (IMN) and, thus, the dose to the heart could potentially be reduced. The aim of this study was to compare proton therapy in FB with proton therapy in DIBH for patients with breast cancer in respect to minimizing heart exposure.Material and Methods
In this study, 16 patients with left-sided breast cancer receiving loco-regional proton therapy (including the IMN) were included. The FB and DIBH plans were created for each patient using spot-scanning proton therapy with 2-3 en face fields, single field optimization and robust optimization. All plans were created with a prescription dose of 40 Gy relative biological effectiveness (RBE) in 15 fractions. To reduce treatment delivery time for the DIBH plans, minimum monitor unit per spot and the distance between spots was increased. The magnitude of the caudal shift of the heart in DIBH was measured as the longitudinal difference in heart position relative to the IMN CTV on the CT scans in FB and DIBH.Results
All plans complied with target coverage constraints. The median MHD was statistically significant reduced from 1.1 to 0.6 Gy RBE by applying DIBH. The median caudal shift of the heart in DIBH was 2.7 cm (range, -0.7 cm to 5.2 cm) which may explain the reduced MHD in the DIBH plans. No statistical significant difference was seen for mean dose and V17Gy RBE to the ipsilateral lung. The median of the treatment delivery time for the DIBH plans was reduced by 27% compared to the FB plans without compromising the plan quality. The estimated number of breath-holds (assuming that patients perform breath-holds of 20 seconds) ranged from 11 to 15 breath-holds with the DIBH plans.Conclusion
Even though the median absolute dose reduction to the heart was limited, it could still be relevant to consider DIBH for a subset of these patients with the largest reduction in mean dose. However, a large caudal shift of the heart in DIBH does not necessarily imply a large reduction of dose to the heart compared with FB, and, thus, comparative proton treatment planning in FB and DIBH will be needed to identify patients with the largest dosimetric benefit.Speaker: Line Bjerregaard Stick (Danish Centre for Particle Therapy) -
16:30
Towards automated prompt-gamma treatment verification: Feasibility of PGI simulations on cone-beam CTs 2h 30mSpeaker: Stefanie Bertschi
-
16:30
-
19:06
→
19:25
Bus PSI dep 19:06 -> Brugg arr 19:25 19m
-
19:30
→
22:30
Dinner / Social Night 3h
-
08:25
→
08:45
-
-
08:30
→
14:00
Joint 4D Workshop & RAPTOR School
-
08:35
Bus Brugg dep. 08:35 -> PSI 08:50 10m
-
08:45
Welcome & Registration (RAPTOR) 25m WHGA/001 (PSI)
WHGA/001
PSI
-
09:10
Experiences from 4D photons: Online adaptive therapy with Linacs 40m WHGA/001
WHGA/001
PSI
Speaker: Dr Stephanie Tanadini-Lang (University Hospital Zurich) -
09:50
Experiences from 4D photons: 4D dose painting 40m WHGA/001
WHGA/001
PSI
Speaker: Dr Olga Sokol (GSI) -
10:30
Coffee break 20m WHGA/001 (PSI)
WHGA/001
PSI
-
10:50
Future of real-time adaptive: 4DMRI 40m WHGA/001
WHGA/001
PSI
Speaker: Prof. Martin Fast (University Medical Center Utrecht) -
11:30
Future of real-time adaptive: AI for 4D 40m WHGA/001
WHGA/001
PSI
Speaker: Dr Ye Zhang (PSI - Paul Scherrer Institut) -
12:10
Future of real-time adaptive: MR-PT 40m WHGA/001
WHGA/001
PSI
Speaker: Prof. Aswin Hoffmann (OncoRay) -
12:50
Closing with poster awards 10m WHGA/001
WHGA/001
PSI
-
13:00
Lunch 1h WHGA/001 (PSI)
WHGA/001
PSI
-
08:35
-
08:30
→
14:00
