12th International Workshop on Radiation Damage to Biological Samples
Auditorium
Paul Scherrer Institut
Thank You for Making RD12 a Remarkable Success
We extend our heartfelt thanks to everyone who contributed to the success of the 12th International Workshop on X-ray Radiation Damage to Biological Samples (RD12) at the Paul Scherrer Institute.
Your engagement—whether as a speaker, poster presenter, participant, sponsor, or organizer—was at the heart of what made RD12 such a vibrant and inspiring event. Over the course of three days, the exchange of ideas across disciplines, institutions, and countries created a dynamic forum that truly advanced the field of X-ray radiation damage research.
We’re especially grateful to our invited speakers and poster contributors for sharing their latest findings, and to the Swiss Light Source and SwissFEL teams for the engaging facility tours. The workshop would not have been possible without the dedication of the organizing committee and the generous support of our sponsors.
We look forward to continuing this momentum through the upcoming special issue in Acta Crystallographica Section D, and we’re already excited for what RD13 will bring.
Thank you for being part of the RD12 community—your presence made it unforgettable.
Download the corrected Book of Abstracts here:
-
-
12:00
→
13:00
Catering: Registarion & Welcome Lunch Auditorium Entrance
Auditorium Entrance
Paul Scherrer Institut
-
13:00
→
13:15
General: Welcome and Introduction Auditorium
Auditorium
Paul Scherrer Institut
Forschungsstrass 111 5232 Villigen PSI SwitzerlandConvener: Elspeth GARMAN (University of Oxford) -
13:15
→
15:15
Biological Studies Affected by Radiation Damage: BIO 1 Auditorium
Auditorium
Paul Scherrer Institut
Forschungsstrass 111 5232 Villigen PSI SwitzerlandConvener: Kunio HIRATA (RIKEN)-
13:15
Rejuvenating online UV-Vis microspectrophotometry by monitoring dose- and time-resolved phenomena at both cryogenic and room temperature 30m
The French protein crystallography beamline BM07-FIP2 at the ESRF enables both cryogenic and room-temperature studies on single crystals, with precise control over the deposited X-ray dose thanks to a large, homogeneous top-hat beam. [1] In addition, its sample environment allows for easy integration of the EMBL/ESRF microspectrophotometer [2], enabling in crystallo UV-Visible absorption and fluorescence measurements in parallel with X-ray diffraction. This approach has allowed for the monitoring of the evolution of the absorbance of metal centres, cofactors, or chromophores as a function of X-ray dose, providing real-time insights into both the extent of radiation damage and the functional state of macromolecules. This presentation will focus on recent methodological developments that led studying the extent of radiation damage on various proteins, enabling the comparison between room and cryogenic temperature. Finally, the plans for the future sample environment of BM07-FIP2 fully integrating an improved microspectrophotometer will be described.
References
[1] McCarthy A. et al. (2025) J Synchrotron Radiat., 32, in press.
[2] McGeehan J, Ravelli RB, Murray JW, Owen RL, Cipriani F, McSweeney S, Weik M, Garman EF. (2009) J Synchrotron Radiat., 16, 163-172.Speaker: Sylvain ENGLIBERGE (IBS Grenoble) -
13:45
Specific Radiation Damage to Halogenated Inhibitors and Ligands in Protein-Ligand Crystal Structures 30m
Protein–inhibitor crystal structures aid medicinal chemists in efficiently improving the potency and selectivity of small-molecule inhibitors. It is estimated that a quarter of lead molecules in drug discovery projects are halogenated. Protein–inhibitor crystal structures have shed light on the role of halogen atoms in ligand binding. They form halogen bonds with protein atoms and improve shape complementarity of inhibitors with protein binding sites. However, specific radiation damage (SRD) can cause cleavage of carbon–halogen (C–X) bonds during X-ray diffraction data collection. This study shows significant C–X bond cleavage in protein–ligand structures of the therapeutic cancer targets B-cell lymphoma 6 (BCL6) and heat shock protein 72 (HSP72) complexed with halogenated ligands, which is dependent on the type of halogen and chemical structure of the ligand. The study found that metrics used to evaluate the fit of the ligand to the electron density deteriorated with increasing X-ray dose, and that SRD eliminated the anomalous signal from brominated ligands. A point of diminishing returns is identified, where collecting highly redundant data reduces the anomalous signal that may be used to identify binding sites of low-affinity ligands or for experimental phasing. Straightforward steps are proposed to mitigate the effects of C–X bond cleavage on structures of proteins bound to halogenated ligands and to improve the success of anomalous scattering experiments.
References
[1] Rodrigues, M.J., Cabry, C., Collie, G., Carter, M., McAndrew C., Owen, R.L., Bellenie, B.R., Le Bihan, Y-V., van Montfort, R.L.M. (2024) J. Appl. Cryst., 57, 1951-1965.Speaker: Matthew J. RODRIGUES (Diamond) -
14:15
Capturing X-ray-Induced Photo-Reduction in Arsenite Oxidase: Implications for the Catalytic Mechanism 30m
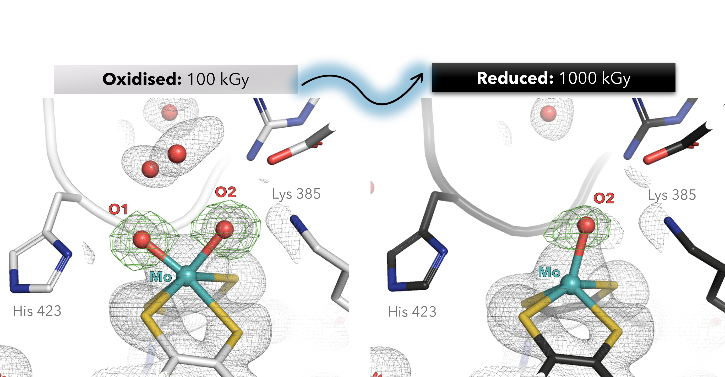
The molybdenum enzyme arsenite oxidase (Aio), is a promising biocatalyst for the detoxification and biosensor applications [1]. So far, its structural characterization and complete reaction mechanism understanding has been limited to artifactually reduced states of the Mo cofactor (Moco) caused by X-ray photoreduction (PDB 1g8k, 4aay) [2-4].
Here, we present the first crystallographic evidence of X-ray-induced photo-reduction in Alcaligenes faecalis Aio, tracking active-site geometric changes across incremental radiation doses (100–1000 kGy) at the PETRA III P14 beamline. High-resolution structures (1.5 Å) reveal that photo-reduction triggers the loss of a labile oxo ligand (O1), while displacing the Mo atom toward the dithiolene plane.
Low-dose data (100 kGy) allowed us to capture, for the first time, the Mo(VI) centre in a six-coordinated geometry with asymmetric cis-dioxo coordination (Mo–O1: 1.8 Å; Mo–O2: 2.1 Å). Reduction of the Moco also flattened the twist angle of the pterin from 31° to 17°, modulating the active site catalysis.
Our work demonstrates that radiation damage artifacts—prevalent in metalloenzyme crystallography—can obscure mechanistic insights. These findings underscore the need for dose-optimized structural data to refine AI-driven models of metalloenzyme mechanisms and advance the rational engineering of Aio for biosensing and bioremediation.
References
[1] Male, K.B. et al. (2007) Anal. Chem, 79(20), 7831–7837.
[2] Warelow T.P. et al., (2013) PLoS One, 8(8): e72535.
[3] Ellis, P.J. et al. (2001) Structure, 9(2), 125-132.
[4] Engrola, F. et al. (2023) J. Biol. Chem, 299(8), 105036.Speaker: Filipa ENGROLA (UCIBIO) -
14:45
Diffraction Intensity as a Radiation Damage Progression Metric and Intensity Decay Models 30m
Ever since the first systematic study of radiation damage in macromolecular crystallography, diffraction intensity decay has been useful as one of the metrics to monitor the progression of radiation damage in reciprocal space for room temperature [1] and cryo-cooled (~100 K) [2] crystalline samples. Various models have been used to parameterise the functional form of the intensity decay (IDMs), but none thus far have been completely satisfactory in both fitting the data and having physically interpretable parameters. The absorbed dose is the most useful x-axis against which to plot intensity decay, since it allows different experimental arrangements to be compared, and the program RADDOSE-3D [3,4] was developed to allow convenient dose estimation.
Results from the recent inclusion of the option to enter an IDM [5] into RADDOSE-3D will be presented. The IDM enables the previous RADDOSE-3D output of ‘Diffraction Weighted Dose’ to be modified from a ‘Fluence Weighted Dose’ to a ‘Diffraction-Decay Weighted Dose’ [6], allowing more informed decisions to be made on possible data collection strategies. Over the last 10 years RADDOSE-3D [4] has been extended for use in a wide variety of diffraction and scattering experiments (MX, SMX, XFEL, SAXS, XPS, PXRD) and most recently, electron diffraction, RADDOSE-ED [6]. In addition, we have now released a new RADDOSE-3D GUI [6,7] which allows the estimation of dose for any of these modalities.
References
[1] Blake, C.C.F., Phillips, D.C. In Proceedings of the Symposium on the Biological Effects of Ionising Radiation at the Molecular Level (Vienna: International Atomic Energy Agency), (1962) pp. 183–191
[2] Gonzales, A., Nave, C. Acta Cryst. D (1994) 50, 874-877
[3] Zeldin, O,B., Gerstel, M., Garman, E.F. J. Appl. Cryst (2013) 46, 1225-1230
[4] Bury, C.S., Brooks-Bartlett, J.C., Walsh, S.P., Garman, E.F. Protein Science (2018) 27, 217–228
[5] Leal, R.M.., Bourenkov, G., Russi, S., Popov, A.N. J Synchrotron Radiat. (2013) 20(1),14–22
[6] Dickerson, J.L., McCubbin, P.T.N., Brooks-Bartlett, J.C., Garman, E.F. Protein Science (2024) 33:e5005.
[7] https://github. com/GarmanGroup/RADDOSE-3DSpeaker: Elspeth GARMAN (University of Oxford)
-
13:15
-
15:15
→
15:45
Catering: Coffee Auditorium Entrance
Auditorium Entrance
Paul Scherrer Institut
-
15:45
→
17:45
Damage at New Sources - XFELs and 4th Generation Synchrotrons Auditorium
Auditorium
Paul Scherrer Institut
Forschungsstrass 111 5232 Villigen PSI SwitzerlandConvener: Valérie PANNEELS (PSI - Paul Scherrer Institut)-
15:45
A protein switch to bind different redox states in the cyanobacterial FutA iron binding protein revealed by an X-ray pump-probe approach 30m
Radiation damage is a faithful attender to X-ray crystallographic studies of metallo-proteins. Thus, care is taken to limit the effects of radiation damage and avoid consequent misinterpretation of X-ray crystallographic results. In our study, we applied defined doses to selectively probe the redox states involved in metal binding.
We studied the cyanobacterial iron binding protein FutA, an ABC transporter substrate binding protein that can also act as an intracellular iron binding protein [1]. We determined crystallographic structures using X-ray and neutron radiation characterised as ferrous [Fe(II)] and ferric [Fe(III)] complexes [2]. These states are distinguished by protein conformation, particularly the positioning of the positively charged Arg203 side chain as part of the iron binding site in the [Fe(II)] complex.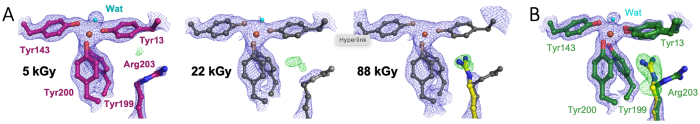.png)
We captured the transition between [Fe(III)] and [Fe(II)] states upon X-ray photoreduction with a dose series using a serial synchrotron crystallography fixed target approach, see panel A. Using a novel XFEL X-ray pump-probe approach, we uncovered how Arg203 functions as a molecular switch, enabling accommodation of different iron oxidation states, see panel B [2]. The switching capability of the single FutA protein provides functional insight and suggests genome streamlining, where the loss of specialised FutA variants may reflect ecological adaptation.
References
[1] Polyviou, D. et al. (2018) J. Biol. Chem., 293, 18099-18109.
[2] Bolton, R. et al. (2024) PNAS, 121, e2308478121.Speaker: Ivo TEWS (University of Southampton) -
16:15
Radiation Damage Aspects in Ultra-High-Resolution Single-Crystal MX 30m
solely deciphering structures and refining atomic positions, to the analysis of subtle structural effects. Examples involve unpredicted deviations in molecular geometries; the details of protonation states of functional groups, ligands and solvent molecules; occupancies of mixed states; atomic vibrations and, finally, electron density studies of active sites and ligands. Classical high-resolution cryogenic single-crystal MX remains an essential complement to time-resolved methods. The underlying data collection methodologies, in which radiation damage continues to be the most important consideration, are evolving from being able to predict diffraction half-life times towards providing optimal choices both delivering the highest data precision and preserving the interpretability of subtle structural features. In practice, virtually every such feature must be experimentally tested to exclude radiation damage artifacts.
The use of large, homogeneous beams at high photon energies up to 30 keV, in combination with high-Z detectors and advanced multi orientation data collection strategies [1, 2] are key. Given that ultra-high resolution diffraction experiments rely on the use of large, coherently diffracting crystals these experiments necessarily require large, top-hat X-ray beams. This is also a prerequisite for precise and reproducible X-ray dosage. We will review the instrumental aspects involved in creating and using such beams at modern beamlines, as well as the possibilities offered by the potential of fourth-generation synchrotron sources.
Finally, we will present the analysis of dose series collected on several ultra-high-resolution structures, both in reciprocal and in real space, in conjuction with previously proposed radiation-damage models [3, 4, 5].References
[1] Donath, T. et al. (2023) J. Synchrotron Rad., 30, 723-738.
[2] Oscarsson, M. et al. (2019) J. Synchrotron Rad. 26, 393–405.
[3] Bourenkov, G. et al., (2006). The 4th International Workshop on X-ray Damage to Biological Crystalline Samples, SPring-8, Japan.
[4] Bourenkov, G. P. & Popov, A. N. (2010). Acta Cryst. D66, 409–419.
[5] Leal, R. et al., J. Synchrotron Rad. (2013). 20, 14–22.Speaker: Gleb BOURENKOV (EMBL Hamburg) -
16:45
Investigation of radiation damage in room temperature serial macromolecular crystallography at a fourth generation Synchrotron 30m
Radiation damage is major concern in macromolecular crystallography (MX) where ionising X-rays used for structure determination can result in a cascade of damaging processes caused by the absorption of energy (denoted mainly as dose: absorbed energy/mass (J/kg; Gray (G)) and radiolysis of molecules. These can introduce artefacts within the structure (specific damage) and the overall data (global damage). Collection at cryogenic temperatures (100 K) is normally performed to mitigate the effects of radiation damage but recently there has been a resurgence in room temperature (RT) and serial crystallography collection. Serial synchrotron crystallography (SSX), with approaches adopted from X-ray free electrons lasers (XFELs), provides a suitable way to collect RT data at synchrotrons with reduced radiation damage by spreading the total dose over thousands of individual microcrystals. This usually relies on a microfocus beamline and a very low-dose per crystal collection strategy to compensate.
With advancements in technologies, synchrotrons are now being upgraded or constructed to fourth generation light sources, offering much higher brilliance compared to their predecessors, and, consequently, microfocus MX beamlines with increased flux density are becoming more routinely available offering much higher dose rates for RT-SSX collection. One example of this is ID29, the flagship beamline constructed at the high-energy fourth generation European Synchrotron Radiation Facility (ESRF), following its upgrade to the Extremely Brilliant Source (ESRF-EBS) [Raimondi et al, 2023]. ID29 is currently in unique territory, delivering slightly polychromatic (1% ΔE/E) microsecond X-ray pulses (90 µs) with a flux density of > 1014 ph/s/µm2, three times higher than third generation sources, and with dose rates on the order of several GGy/s. The unique beam characteristics on ID29 allow for the possibility of serial microsecond crystallography (SµX) for the structure determination of macromolecules using RT-SSX [Orlans et al, 2025]. As ID29 is setting a precedent for similar beamlines that are appearing or due to appear worldwide, a comprehensive radiation damage analysis is thus required.
References
[1] Raimondi et al. (2023) Communications Physics, 6, 82.
[2] Orlans et al. (2025) Communications Chemistry, 8, 6.Speaker: Samuel L. ROSE (ESRF) -
17:15
Damage before destruction? X-ray-induced changes in single-pulse serial femtosecond crystallography 30m
Serial femtosecond crystallography (SFX) exploits extremely brief X-ray free-electron laser pulses to obtain diffraction data before destruction of the crystal. However, during the pulse X-ray-induced site-specific radiation damage can occur, leading to electronic state and/or structural changes. Here, we present a systematic exploration of the effect of single-pulse duration and energy (and consequently different dose rates) on site-specific radiation damage under typical SFX room-temperature experimental conditions. For the first time in SFX we directly measured the photon pulse duration, varying from less than 10 fs to more than 50 fs, and used three pulse energies to probe in-pulse damage in two radiation-sensitive proteins: the iron-heme peroxidase DtpAa and the disulfide-rich thaumatin. While difference-map features arising from radiation damage are observed, they do not lead to significant change in refined atomic coordinates or key bond lengths. Our work thus provides experimental verification that average atomic coordinates are not significantly perturbed by radiation damage in typical SFX experiments.
Speaker: Robin OWEN (Diamond Light Source)
-
15:45
-
17:45
→
18:15
Posters: Poster Blitz Auditorium
Auditorium
Paul Scherrer Institut
Forschungsstrass 111 5232 Villigen PSI SwitzerlandConvener: Florian DWORKOWSKI (PSI - Paul Scherrer Institut) -
18:15
→
19:00
Discussion Auditorium
Auditorium
Paul Scherrer Institut
Forschungsstrass 111 5232 Villigen PSI SwitzerlandConveners: Michael HOUGH (University of Essex), Nadia ZATSEPIN -
19:00
→
20:00
Catering: Dinner Auditorium Entrance
Auditorium Entrance
Paul Scherrer Institut
-
20:00
→
21:30
Posters: Posters & Drinks Auditorium
Auditorium
Paul Scherrer Institut
Forschungsstrass 111 5232 Villigen PSI Switzerland
-
12:00
→
13:00
-
-
09:00
→
10:00
Biological Studies Affected by Radiation Damage: BIO 2 Auditorium
Auditorium
Paul Scherrer Institut
Forschungsstrass 111 5232 Villigen PSI SwitzerlandConvener: Edward SNELL (Hauptman-Woodward Medical Research Institute)-
09:00
X-ray photoreduction of the active site copper in the fungal lytic polysaccharide monooxygenase LsAA9A 30m
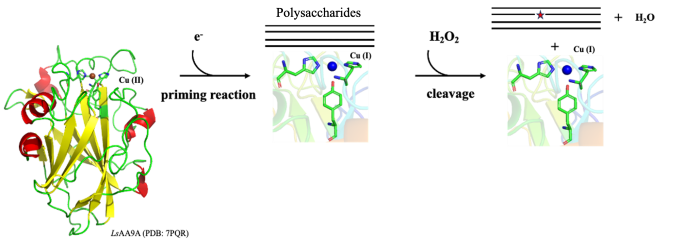
Lytic polysaccharide monooxygenases are enzymes [1] binding their active-site copper through the characteristic His-brace motif shown above including two His – one N-terminal – and often also a Tyr. The reaction cycle starts with reduction of the resting state Cu(II) to Cu(I) – in the laboratory usually using ascorbate as small molecule reductant. Despite the name, most LPMOs prefer hydrogen peroxide as co-substrate, to subsequently oxidatively cleave the glycosidic bonds in saccharides.
We have previously – through crystal cryo-structures of the model enzyme LsAA9A determined at high and low X-ray doses, and based on the hypothesis that X-ray induced photoreduction mimics natural priming reaction - reconstructed possible changes in geometry during the catalytic cycle and identified a small shortening of the Cu(II)-Tyr distance [2].
Aside from uncertainty on the biological significance of such shortening [3], we wanted to address concerns regarding the ability of macromolecular crystallography to reliably detect differences in the order of 0.1-0.2 Å. We thus carried out additional triplicate independent structure determination representing Cu(I)/Cu(II) states with/without the model substrates cellotriose, showing statistically significant differences only for the Cu(II)-Tyr distance with/without saccharide, but no other Cu-protein distance.
In order to assess whether additional general X-ray damage obscures similar shortening in the Cu(I) state induced by photoreduction, we are now comparing with cryo data collected after priming by chemical reduction with ascorbate at low X-ray doses.
Finally since X-ray-induced photoreduction of the active-site copper may closely approximate the chemical priming reaction, it holds potential as a trigger for time-resolved studies. To explore this possibility further, we are currently investigating the photoreduction process at room temperature.References
[1] Tandrup, T., Frandsen, K.E.H., Johansen, K.S., Berrin, J.-G., Lo Leggio, L. (2018) Biochem. Soc. Trans. 46, 1431–1447
[2] Tandrup, T., Muderspach, S.J., Banerjee, S., Santoni, G., Ipsen, J.Ø., Hernández-Rollán, C., Nørholm, M.H.H., Johansen, K.S., Meilleur, F., Lo Leggio, L. (2022) IUCR Journal 9, 666-681.
[3] Wieduwilt, Lo Leggio and Hedegård (2024), Dalton Trans, 53, 5796-5807.Speaker: Leila LO LEGGIO (University of Copenhagen) -
09:30
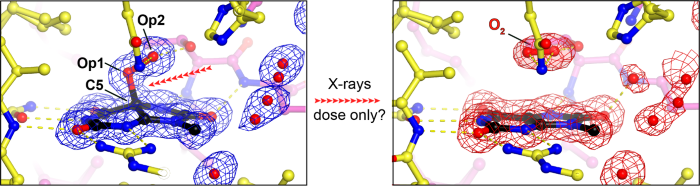
Breaking Bad...or not..: altering experimental conditions to probe bond fate 30m
Urate oxidase (UOX) is the archetypal cofactor-independent oxidase, catalysing the O₂-mediated degradation of uric acid in many organisms. Previously, we demonstrated that the first step of this reaction is a dioxygenation reaction, forming 5-peroxyisourate (5-PIU) as an intermediate [1]. We also showed that 5-PIU is highly sensitive to radiation: during conventional single-axis X-ray diffraction experiments at 100 K, doses between 50-100 kGy caused visible damage to the C5–Op1 bond, and doses around 200 kGy led to complete bond rupture, accompanied by in situ release of O₂ [1,2]. Surprisingly, a subsequent synchrotron serial crystallography (SSX) experiment at room temperature (RT) showed no damage to this bond, even at an estimated dose of up to 70 kGy [3]. In this talk, I will discuss our more recent experiments exploring radiation-induced bond rupture under various experimental conditions—including gaussian vs. top-hat beam profiles, cryogenic vs. room temperature, and serial vs. conventional crystallography—in an effort to better connect these observations to the reaction mechanism.
.png)
References
[1] Bui S, von Stetten D, Jambrina PG, Prangé T, Colloc'h N, de Sanctis D, Royant A, Rosta E, Steiner RA (2014), Angew Chem Int Ed, 53, 13710-13714.
[2] Bui S and Steiner RA (2017) Curr Opin Struct Biol, 41,109-118.
[3] Zielinski KA, Prester A, Andaleeb H, Bui S, Yefanov O, Catapano L, Henkel A, Wiedorn MO, Lorbeer O, Crosas E, Meyer J, Mariani V, Domaracky M, White TA, Fleckenstein H, Sarrou I, Werner N, Betzel C, Rohde H, Aepfelbacher M, Chapman HN, Perbandt M, Steiner RA, Oberthuer D (2022) IUCrJ, 31:778-791.Speaker: Roberto STEINER (King's College London / University of Padua)
-
09:00
-
10:00
→
11:00
Radiation Damage in Electron Crystallography and Microscopy Auditorium
Auditorium
Paul Scherrer Institut
Forschungsstrass 111 5232 Villigen PSI SwitzerlandConvener: Thomas URSBY (MaxIV)-
10:00
TEM and STEM Imaging of Radiation-Sensitive Samples 30m
Beam damage to biological specimens is more troublesome in the TEM than in x-ray imaging (where the spatial resolution is more modest) despite the stronger diffraction signal provided by electrons [1]. One solution has been to treat tissue (and other) specimens with heavy-metal stain but the preference is to use unstained samples and phase contrast, while cooling the sample to reduce damage (cryo-EM).
Whereas the electron optics of a TEM can provide atomic-scale contrast, the image resolution for a beam-sensitive sample is usually limited by the effects of radiolysis. The signal/noise ratio (SNR) in the image is then determined by beam-electron shot noise and the damage-limited resolution is given [2] by(1) $\mathrm{DLR }=(\mathrm{SNR})C^{-1}[(\mathrm{DQE})F D_{c}]^{{-\frac{1}{2}}}$
Optimizing resolution involves paying attention to each term in Eq. (1). SNR is usually taken as 3 or 5 (the Rose criterion) but a large improvement is possible if there are multiple copies of the same object, as in diffraction imaging or single-particle analysis (SPA). The image contrast C is high in dark-field mode but for thin samples the electron-collection efficiency F is low. Phase contrast is efficient but is problematic for thicker samples, as required for most tomographic (3-dimensional) imaging. The detective quantum efficiency (DQE) of the electron detector can be maximized by using direct recording, rather than employing a scintillator.
The characteristic dose Dc represents the maximum fluence that the specimen can withstand before the structure being observed is degraded. Dc can be increased by cooling the sample with liquid nitrogen or liquid helium [3] or by coating it with an evaporated layer, and graphene encapsulation seems to be even more effective
Dose rate seems to have little effect on the radiolysis damage to organic specimens, except for secondary effects (mass loss) at a low specimen temperature and at the high dose rates (>1015 Gy/s) possible in scanning-mode TEM (STEM). Unlike XFEL x-ray imaging, it will likely remain impossible to outrun the primary stage of damage with electrons, due to their electrostatic repusion. Earlier reports of two-fold damage reduction using femtosecond electron pulses have recently been disputed [4].References
[1] Henderson, R. (1995) Q. Rev. Biophys., 28, 171-193.
[2] Egerton, R.F. (2024) Micron, 177, 103576.
[3] Naydenova, K. et al. (2022) Ultramicroscopy, 237, 113512.
[4] Zhao, Y. et al. (2024) bioRxiv preprint: https://10.1101/2024.12.20.629524Speaker: Ray EGERTON (University of Alberta) -
10:30
Extending the reach of single-particle cryoEM 30m
Ten years on from the “resolution revolution”, molecular structure determination using electron cryomicroscopy (cryoEM) is poised in 2025 or early 2026 to surpass X-ray crystallography as the most used method for experimentally determining new structures [1]. But the technique has not reached the physical limits set by radiation damage and the signal-to-noise ratio in individual images of molecules. By examining these limits and recent work on radiation damage to biological molecules at different temperatures [2,3] and energies [4,5], I will identify opportunities for extending the application of single-particle cryoEM to smaller, larger and more difficult structures, and into specimens taken directly from vitrified cells. This will help guide technology development to continue the exponential growth of structural biology in the coming decade.
References
[1] A. Patwardhan, R. Henderson, C.J. Russo, Extending the reach of single-particle cryoEM, COSB in press (2025).
[2] K. Naydenova, A. Kamegawa, M. J. Peet, R. Henderson, Y. Fujiyoshi, C. J. Russo, On the reduction in the effects of radiation damage to two-dimensional crystals of organic and biological molecules at liquid-helium temperature, Ultramicroscopy 237 (2022) 113512.
[3] J. L. Dickerson, K. Naydenova, M. J. Peet, H. Wilson, B. Nandy, G. McMullan, R. Morrison, C. J. Russo. Reducing the effects of radiation damage in cryo-EM using liquid helium temperatures. PNAS 2025
[4] M.J. Peet R. Henderson C.J. Russo, The energy dependence of contrast and damage in electron cryomicroscopy of biological molecules, Ultramicroscopy 203 (2019) 125–131.
[5] G. McMullan et al. Structure determination by cryoEM at 100 keV. PNAS 120 (2023) e2312905120.Speaker: Christopher J. RUSSO (MRC Cambridge)
-
10:00
-
11:00
→
11:30
Catering Auditorium Entrance
Auditorium Entrance
Paul Scherrer Institut
-
11:30
→
13:30
Radiation Damage in Electron Crystallography and Microscopy Auditorium
Auditorium
Paul Scherrer Institut
Forschungsstrass 111 5232 Villigen PSI SwitzerlandConvener: Gerhard HOFER (Stockholm University)-
11:30
Perspectives and limitations in high-resolution cryo-EM 30m
Single particle cryo-EM has become a powerful technology in structure determination of macromolecular complexes. The technique benefits from the decades of investment in the development of high-end electron microscopy hardware, powerful computational hard- and software and the fact that samples do not need to be crystalized. Over the past decade, the number of structures solved by cryo-EM has consequently increased and soon a point will be reached when more structures will be solved in academia by cryo-EM than by X-ray crystallography. Simultaneously, the maximum resolution limit of the technology has been improved and at least for the model protein apoferritin even atomic resolution has been achieved [1].
In spite of this success story, the mean resolution of single particle cryo-EM structures deposited in the database (EMDB), is still >4 Angstrom even until today. The important question is therefore, why can this technology potentially obtain even atomic resolution structures and what are the limiting factors that prevent the user from obtaining this level of resolution on a regular basis?
A major limiting factor in cryo-EM is the biochemical quality of a purified macromolecular complex. Macromolecules are known to be prone to suffer in quality during biochemical purification but also during electron microscopical grid preparation and often it isn’t even an easy task to distinguish between the two effects. Other limitations can be attributed to electron optical limitations but also limitations in computational image processing.
Achieving routine atomic resolution depends on parallel improvements in all three pillars of the technology. For example, even a small amount of sample degradation during biochemical purification or grid preparation can negate the gains made by state-of-the-art electron microscopes. Similarly, poor electron optics can limit the usefulness of pristine samples. Overcoming these challenges requires a holistic approach: refining biochemical workflows to preserve macromolecular integrity, optimising electron optics to minimise imaging artefacts, and developing adaptive software pipelines to extract maximum information from complex datasets.References
[1] Yip K.M., Fischer N., Paknia E., Chari A., Stark H. (2020) Nature, Atomic-resolution protein structure determination by cryo-EM, 157-161.
[2] Nakane T, et al. (2020) Nature, Single-particle cryo-EM at atomic resolution. 152-156.Speaker: Holger STARK (MPI Göttingen) -
12:00
A Physical Theory For Cryo-EM at Liquid-Helium Temperatures 30m
The benefits of reducing the data collection temperature for electron cryomicroscopy (cryo-EM) from liquid-nitrogen temperatures to liquid-helium temperatures have been debated over many years. A physical theory of dose-dependent information loss in cryo-EM was presented for imaging vitrified aqueous biological specimens at liquid-nitrogen temperatures [1], but extending this to liquid-helium temperatures is needed. Previously, it was demonstrated that there is a 1.2–1.8x reduction in radiation damage for 2D protein crystals when imaging at temperatures near liquid helium [2]. Unfortunately, lowering specimen temperatures for cryo-EM of macromolecules embedded in vitreous ice has consistently proven to be no better than liquid-nitrogen temperatures and is often worse [3]. We aimed to determine whether the reduction in radiation damage measured in 2D crystals extends to single-particle cryo-EM and, if so, what else could be limiting data quality.
Consequently, we investigated several dose-dependent physical phenomena that could limit single-particle cryo-EM data quality: radiation damage, microscopic charge fluctuations, charge accumulation, pseudo-Brownian motion of water, mass loss, hydrogen bubbling, and beam-induced motion. We found that radiation damage is reduced by a similar amount for single-particle cryo-EM as was measured by 2D crystallography. We demonstrate that the reduction in data quality is likely caused by beam-induced motion, with all other physical phenomena that we measured being either unchanged or not sufficient to cause a reduction in image quality at lower specimen temperatures. Using novel specimen supports, we have been able to eliminate this beam-induced motion and determined cryo-EM structures at liquid-helium temperatures where every frame carries more information compared to the equivalent at liquid-nitrogen temperatures [4]. Alongside the development of new TEMs capable of operating at temperatures below liquid-nitrogen, this theory will enable cryo-EM to resolve smaller molecules than is currently possible.References
[1] R. Henderson, C. J. Russo, Single Particle CryoEM: Potential for Further Improvement, Microscopy and Microanalysis 25 (S2) (2019) 4–5.
[2] K. Naydenova, A. Kamegawa, M. J. Peet, R. Henderson, Y. Fujiyoshi, C. J. Russo, On the reduction in the effects of radiation damage to two-dimensional crystals of organic and biological molecules at liquid-helium temperature, Ultramicroscopy 237 (2022) 113512.
[3] O. Pfeil-Gardiner, D. J. Mills, J. Vonck, W. Kuehlbrandt, A comparative study of single-particle cryo-EM with liquid-nitrogen and liquid-helium cooling, IUCrJ 6 (6) (2019) 1099–1105.
[4] J. L. Dickerson, K. Naydenova, M. J. Peet, H. Wilson, B. Nandy, G. McMullan, R. Morrison, C. J. Russo. Reducing the effects of radiation damage in cryo-EM using liquid helium temperatures. PNAS 2025Speaker: Joshua DICKERSON (UC Berkeley) -
12:30
Cryo-EM structure of photosystem II reveals damages caused by commonly used electron doses in imaging 30m
Photosystem II (PSII) plays a critical role in water-splitting and oxygen evolution. X-ray crystallography has elucidated its atomic structure and structures of several intermediate states. However, these structures are in the crystalline state, and the structure of the final state remains unresolved. In this study, we analyzed the structure of PSII in solution at a resolution of 1.95 Å using single-particle cryo-electron microscopy (cryo-EM). The obtained structure is similar to the crystal structure, but regions susceptible to redox state changes exhibited electron beam damages at high doses. By reducing the number of movie frames of electron micrographs from 50 to 2 to lower the beam dose, the damages were minimized while the resolution was comparable (Fig. a). I will discuss the details of the electron beam-induced damages and their minimization in this presentation.
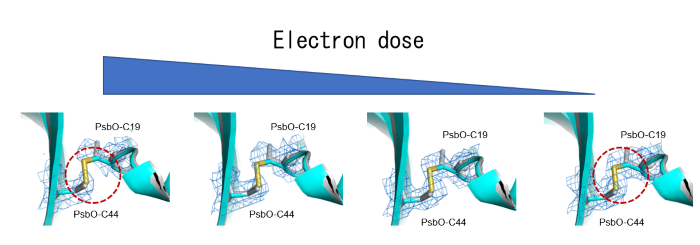.png)
References
[1] Kato K. et al. (2021) Commun Biol., 4, 382.Speaker: Koji KATO (Okayama University) -
13:00
Radiation damage in recent MicroED measurements 30m
Microcrystal electron diffraction (MicroED) involves collecting a sequence of diffraction images from a continuously rotating microcrystal in a transmission electron microscope. Due to the small size of these crystals, the resulting diffraction patterns typically feature weak, low-intensity spots. Recent advances in direct electron-counting detectors have significantly improved signal detection, enabling high-quality data collection at lower electron fluence. This not only shortens acquisition times but also mitigates radiation damage to the sample. Additionally, the introduction of energy filtering has enhanced the accuracy of high-resolution reflection integration without increasing the dose delivered to the sample. In this presentation, we will review early results from radiation damage studies on cryo-cooled crystals of model proteins and highlight recent methodological improvements in MicroED.
Speaker: Johan HATTNE (UCLA)
-
11:30
-
13:30
→
14:30
Catering: Lunch Auditorium Entrance
Auditorium Entrance
Paul Scherrer Institut
-
14:30
→
16:00
General: Visit SLS2.0 and SwissFEL Auditorium
Auditorium
Paul Scherrer Institut
Forschungsstrass 111 5232 Villigen PSI Switzerland -
16:00
→
16:30
Catering: Coffee Auditorium Entrance
Auditorium Entrance
Paul Scherrer Institut
-
16:30
→
18:00
Radiation Damage in Complementary Fields including Biological Imaging Auditorium
Auditorium
Paul Scherrer Institut
Forschungsstrass 111 5232 Villigen PSI SwitzerlandConvener: Daniele DE SANCTIS (ESRF)-
16:30
High-dose effects in high-resolution X-ray microscopy of soft materials 30m
High-resolution X-ray microscopy is used as a complementary approach to electron microscopy for non-destructive imaging of soft materials, including biological tissues, e.g. frozen hydrated [1] or heavy metal stained resin-embedded [2] brain tissues. For this purpose, photon energies above about 2 keV are used to penetrate tissues of about 100 micron thickness or more, for which samples exhibit a very low contrast. To overcome this challenge, phase-contrast hard X-ray microscopy methods are typically used in synchrotron beamlines, reaching a resolution of about 100 nm. The available coherent flux and the changes in the sample structure due radiation are among the main challenges to improve spatial resolution in hard X-ray microscopy of soft materials. Next generation synchrotron sources provide high brilliance, which offers a great opportunity to improve resolution. However, this will require the development of new approaches to mitigate the effects of radiation in soft materials.
Here, we present our experience when applying ptychographic X-ray computed tomography (PXCT) [3] to soft materials. In polymer samples or resin-embedded biological tissues, we observe deformations, such as expansion or contraction of the sample, and mass loss above a certain X-ray dose exposure [4]. For samples that deform during acquisition, we apply a non-rigid tomographic reconstruction to recover the original 3D structure of the specimen [5]. For resin-embedded biological tissues, we have identified a resin which is more resistant to hard X-ray radiation compared to standard epoxy resins used for EM [4]. Finally, we explore acquisition strategies and sample preparation protocols that minimize the effect of radiation.
References
[1] Tran, T.T., et al. (2020) Front. Neurosci. 14, 570019.
[2] Kuan, A.T., et al. (2020) Nat. Neurosci. 23, 1637-1643.
[3] Dierolf, M., et al. (2010) Nature 467, 436-439.
[4] Bosch, C., et al. (2023) bioRxiv, doi: https://doi.org/10.1101/2023.11.16.567403.
[5] Odstrčil, M., et al. (2019) Nat. Commun. 10, 2600.Speaker: Ana DIAZ (Paul Scherrer Institut) -
17:00
Coherent X-rays Reveals Radiation-Induced Dynamics in Hydrated Proteins 30m
Radiation damage remains a central challenge in structural biology, particularly when probing soft, disordered materials like hydrated proteins. In this presentation, I will discuss recent advances using X-ray Photon Correlation Spectroscopy (XPCS) to study radiation-driven dynamics in both hydrated protein powders [1] and dense protein solutions [2,3]. At cryogenic temperatures, we investigate lysozyme powders and find that X-ray exposure induces nanoscale stress relaxation, revealing a temperature-dependent transition around 227 K associated with enhanced dynamical heterogeneity. These results highlight how radiation can stimulate out-of-equilibrium processes in supercooled, granular biomaterials. At ambient temperatures, using megahertz XPCS at the European XFEL, we probe antibody and ferritin solutions as a function of X-ray dose and dose rate. Our measurements capture anomalous diffusion and aggregation dynamics, which are influenced by both hydrodynamic and direct protein interactions. Modeling these effects allows us to disentangle intrinsic molecular behavior from radiation-induced perturbations. Together, these studies underscore the dual role of coherent X-rays as both probe and stimulus, offering critical insights into how radiation impacts biological materials under experimentally relevant conditions. This understanding is essential for optimizing measurement strategies in next-generation X-ray facilities and for minimizing radiation effects in XPCS studies of biological samples.
References
[1] M. Bin et al., Coherent X-ray Scattering Reveals Nanoscale Fluctuations in Hydrated Proteins, J. Phys. Chem. B 127, 4922 (2023).
[2] M. Reiser et al., Resolving molecular diffusion and aggregation of antibody proteins with megahertz X-ray free-electron laser pulses, Nat. Commun. 13, 1 (2022).
[3] A. Girelli et al., Coherent X-Rays Reveal Anomalous Molecular Diffusion and Cage Effects in Crowded Protein Solutions, arXiv:2410.08873.Speaker: Foivos PERAKIS (Stockholm University) -
17:30
Radiation damage in time-resolved X-ray solution scattering experiments 30m
Time-resolved pump-and-probe X-ray solution scattering experiments can probe
kinetics and dynamics of biological macromolecules in real time and under nearnative
conditions. A concern is radiation damage to the sample. One approach to
reduce radiation damage is to use mechanical devices to probe only with short X-ray
pulses. We used this approach to monitor intermediate states in calcium transport at
beamline ID09 at the ESRF 1,2. However, such experimental designs are delicate and
typically only available at dedicated time-resolved synchrotron beamlines, which
limits availability. An alternative approach is to use detector readout to obtain the
temporal resolution, which requires careful characterization of radiation damage. We
developed such an approach at the CoSAXS beamline at MAX IV Laboratory and
tracked kinetics and structural dynamics of the enzyme adenylate kinase 3.References
[1] Ravishankar, H., Pedersen, M.N., Eklund, M., Sitsel, A., Li, C., Duelli, A., Levantino, M., Wulff, M.,
Barth, A., Olesen, C., Nissen, P., Andersson, M. (2020) Science Advances. 6(12), eaaz0981.
[2] Prabudiansyah, I., Orädd, F., Magkakis, K., Pounot, K., Levantino, M., Andersson, M. (2024)
Science Advances. 10(41), eadp2916.
[3] Magkakis, K., Orädd, F., Ahn, B., Da Silva, V., Appio, R., Plivelic, T., Andersson, M. (2024)
Structure. 32(9), P1519-1527.Speaker: Magnus P. ANDERSSON (Umea University)
-
16:30
-
18:00
→
18:45
Discussion Auditorium
Auditorium
Paul Scherrer Institut
Forschungsstrass 111 5232 Villigen PSI SwitzerlandConveners: Briony YORKE, James HOLTON -
18:45
→
19:30
General: Transfer to Brugg Auditorium
Auditorium
Paul Scherrer Institut
Forschungsstrass 111 5232 Villigen PSI Switzerland -
19:30
→
22:30
Catering: Conference Dinner FHNW Windisch
FHNW Windisch
-
09:00
→
10:00
-
-
09:00
→
10:30
Pump-Laser Excitation Conditions in Time-Resolved Serial Femtosecond Crystallography Auditorium
Auditorium
Paul Scherrer Institut
Forschungsstrass 111 5232 Villigen PSI SwitzerlandConvener: Philip JOHNSON (PSI - Paul Scherrer Institut)-
09:00
Ultrafast structural changes in myoglobin: influence of pump laser fluence 30m
The high-intensity femtosecond pulses generated by X-ray free-electron lasers enable pump-probe studies of electronic- and nuclear changes during light-induced reactions. On time scales from femtoseconds to milliseconds and for a variety of biological systems, time-resolved serial femtosecond crystallography (TR-SFX) has provided detailed structural data on processes such as light-induced isomerization, breakage or formation of chemical bonds and electron transfer. However, to date, most if not all ultra-fast TR-SFX studies have employed such high pump laser energies that nominally, several photons were absorbed for each chromophore. As such multiphoton absorption processes may force the protein response into nonphysiological pathways, this is of considerable concern as it poses the question whether this experimental approach allows valid inferences to be drawn about biological processes, which are likely single-photon.
Here we describe an ultrafast pump-probe SFX study of the photodissociation of carboxymyoglobin, which shows that different pump laser fluences result in strikingly different dynamics. In particular, these concern the mechanistically important coherent oscillations of the Fe-CO bond distance (predicted by recent quantum wavepacket dynamics) which are seen to depend strongly on pump laser energy. While our results confirm both the feasibility of performing TR-SFX pump probe experiments in the linear photoexcitation regime, they also show the necessity of doing so. We propose this to be a starting point for the reassessment of the design and interpretation of ultrafast TR-SFX pump probe experiments, to ensure any emergent insights are biologically relevant.Speaker: Thomas R.M. BARENDS (Max Planck Institute for Medical Research) -
09:30
Time-resolved serial femtosecond crystallography on the fluorescent protein rsEGFP2-V151A in different photon excitation regimes 30m
Time-resolved serial femtosecond crystallography (TR-SFX) enables the visualization of ultrafast structural changes in crystalline macromolecules [1]. For light-sensitive proteins, optical excitation lasers represent a convenient means to trigger the reaction. Generally, high excitation-laser fluences are used to maximize light-induced features in the Fourier difference electron density maps [2,3]. However, these fluences generally correspond to nominally more than one absorbed photon per chromophore on average, significantly increasing the risk of unwanted multiphoton effects that convolute with the functionally-meaningful single-photon process of interest [4]. Hence, the choice of excitation laser fluence is a topic of intense discussion in the TR-SFX field [5-7] and a first systematic study on myoglobin indeed evidenced different CO dissociation mechanisms in the single- and the multi-photon regimes [4].
Here, TR-SFX experiments were conducted on the V151A variant of the reversibly photoswitchable fluorescent protein rsEGFP2 [8]. The off- (trans chromophore) to on-state (cis chromophore) photoswitching process was probed at two different time delays (1 and 500 ps) following 150-femtosecond excitation at low, medium and high fluences (0.05, 0.15 and 0.5 mJ/mm² at the Gaussian peak, respectively) corresponding nominally to 0.8, 2, and 8 absorbed photons per chromophore on average. At the high fluence, only a marginal further increase is observed in light-induced Fourier difference electron density peaks compared to the medium fluence, and the expected cis conformer is either absent (1 ps) or occupied below expectation (500 ps). Fluence-dependent time-resolved absorption spectroscopy suggested a chromophore radical species only formed at the high fluence. Our findings suggest that multi-photon induced radical formation at high fluence alters the functional photoisomerization process in rsEGFP2.References
[1] Barends, T.R.M. et al. (2022). Nat Rev Methods Primers, 2.
[2] Brändén, G. & Neutze, R. (2021). Science, 323.
[3] Besaw J.E. & Miller R.J.D (2023). Curr. Opin. Struct. Biol., 81.
[4] Barends, T.R.M. et al. (2024). Nature, 626.
[5] Heyne, K. et al. (2024), Structure, 32.
[6] Neutze, R. & Miller R.J.D. (2024). Nature, 626.
[7] Bertand, Q et al., (2024), Nat. Commun. 15.
[8] Adam, V. et al., (2022), Chem. Phys. Chem, 23.Speaker: Nicolas COQUELLE (CNRS Grenoble) -
10:00
Structural effects of high laser power densities on an early bacteriorhodopsin photocycle intermediate 30m
Time-resolved serial crystallography at X-ray Free Electron Lasers offers the opportunity to observe ultrafast photochemical reactions at the atomic level. The technique has yielded exciting molecular insights into various biological processes including light sensing and photochemical energy conversion. However, to achieve sufficient levels of activation within an optically dense crystal, high laser power densities are often used, which has led to an ongoing debate about the extent to which photodamage may compromise the interpretation of the results. Here we compare time-resolved serial crystallographic data of the bacteriorhodopsin K-intermediate collected at laser power densities ranging from 0.04 to 2493 GW/cm² and follow energy dissipation of the absorbed photons logarithmically from picoseconds to milliseconds. Although the effects of high laser power densities on the overall structure are small, in the upper excitation range we observe significant changes in retinal conformation and increased heating of the functionally critical counterion cluster. We compare light-activation within crystals to that in solution and discuss the impact of the observed changes on bacteriorhodopsin biology.
References
[1] Bertrand, Q. et al. Structural effects of high laser power densities on an early bacteriorhodopsin photocycle intermediate. Nature Communications 15, 10278 (2024).Speaker: Jörg STANDFUSS (PSI - Paul Scherrer Institut)
-
09:00
-
10:30
→
11:00
Catering: Coffee Auditorium Entrance
Auditorium Entrance
Paul Scherrer Institut
-
11:00
→
12:00
Radiation Damage in Temperature Controlled Crystallography Auditorium
Auditorium
Paul Scherrer Institut
Forschungsstrass 111 5232 Villigen PSI SwitzerlandConvener: Vincent OLIERIC (PSI - Paul Scherrer Institut)-
11:00
Mapping the Conformational Landscapes of Human Kinases with Multi-Temperature X-ray Crystallography 30m
Protein kinases are a large family of enzymes that regulate diverse cellular processes by transfering phosphate groups from ATP to their protein substrates. The enzymatic kinase domain (KD) represents a conserved fold, and KD phosphorylation, as well as interactions with cis-regulatory elements and other allosteric effectors, modulates the populations of catalytically active and inactive states present in the KD conformational ensemble. In this presentation, I will briefly describe two studies where multi-temperature X-ray crystallography has provided new insight into the conformational landscapes of two human kinases. In one study, we used determined crystal structures of CDK2, a kinase that regulates the cell cycle and is considered a „white whale“ of drug discovery, across a broad range of temperatures from cryogenic to physiological, to identify inactive conformations that could be targeted to create more selective inhibitors that avoid off-target effects. In the other study, we determined crystal structures of CLK1 across a narrow range of physiological temperatures to understand how this kinase acts as an acute body temperature sensor. Because these studies involve extensive data collection at non-cryogenic temperatures, careful consideration must be given to mitigating the effects of radiation damage.
Speaker: Michael THOMPSON (UC Merced) -
11:30
Exploring Ligand-Protein Interactions at Multiple Temperatures Using Macromolecular Crystallography 30m
Cryogenic temperatures may introduce artefacts that limit the understanding of protein dynamics, crucial to their biological functions. To address this, we developed a room-temperature (RT) X-ray crystallographic method that captures movie-like structural snapshots triggered by temperature [1]. This method revealed binding-mode changes of TL00150, a 175.15 Da fragment, in endothiapepsin. Building on this, we further developed Cryo2RT, a high-throughput RT data-collection method using cryo-cooled crystals, which leverages the cryo-crystallography workflow [2]. This method has been applied to endothiapepsin with four soaked fragments, thaumatin, and SARS-CoV-2 3CLpro, Cryo2RT uncovered distinct ligand-binding modes at RT, not seen at cryogenic temperatures. To minimize radiation damage, X-ray doses were controlled below 500 kGy, a threshold considered safe for both cryo and RT crystallography. Despite similar doses, RT datasets showed slightly lower resolution and higher B-factors (30–40 Ų vs. ~20 Ų at cryo), likely due to increased atomic motion at RT. These findings provide insights into structural interpretation at RT and highlight Cryo2RT's potential for fragment-based screening and studying temperature-dependent dynamics.
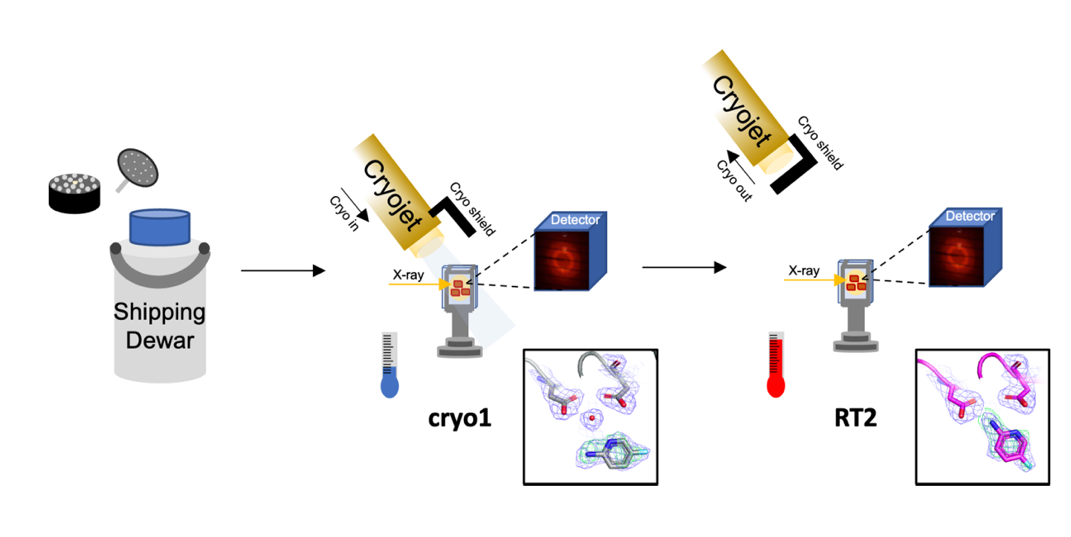
References
[1] Huang, C.-Y., Aumonier, S., Engilberge, S., Eris, D., Smith, K. M. L., Leonarski, F., Wojdyla, J. A., Beale, J. H., Buntschu, D., Pauluhn, A., Sharpe, M. E., Metz, A., Olieric, V., and Wang, M. (2022) Acta Cryst. D78: 964-974. https://doi.org/10.1107/S205979832200612X
[2]: Huang, C.-Y., Aumonier, S., Olieric, V., and Wang, M. (2024) Acta Cryst. D80: 620-628. https://doi.org/10.1107/S2059798324006697Speaker: Chia-Ying HUANG (PSI - Paul Scherrer Institut)
-
11:00
-
12:00
→
12:45
Discussion Auditorium
Auditorium
Paul Scherrer Institut
Forschungsstrass 111 5232 Villigen PSI SwitzerlandConveners: Arwen PEARSON, Colin NAVE -
12:45
→
13:00
General: Summary and Farewell Auditorium
Auditorium
Paul Scherrer Institut
Forschungsstrass 111 5232 Villigen PSI SwitzerlandConvener: Martin WEIK (IBS) -
13:00
→
14:00
Catering: Lunch and Farewell Auditorium Entrance
Auditorium Entrance
Paul Scherrer Institut
-
09:00
→
10:30