Speaker
Description
Magnetoencephalography (MEG) based on optically pumped magnetometers (OPM-MEG) is a relatively novel method to measure brain activity non-invasively in humans. It offers several advantages over traditional SQUID (superconducting quantum interference device) based MEG and EEG [1-3]. However, its properties, in particular test-retest reliability (stability of measurements in time) and construct validity (agreement of results with established technologies), need to be evaluated to make day-to-day, routine applications possible.
In this quality control study, we investigated the reliability and validity of auditory mismatch negativity (MMN) recordings from a newly installed OPM-MEG system (Cerca Magnetics Limited, Nottingham, UK) utilizing 64 triaxial sensors (QuSpin, Louisville, CO, USA). The auditory MMN is an electrophysiological response to rule violations in auditory input streams [4], which has been interpreted as reflecting the update of a predictive (generative) model of the acoustic environment [5]. We recorded OPM-MEG from 30 healthy volunteers, measured twice within 24-72 hours, using an established auditory MMN paradigm [6]. First, we focused on construct validity and investigated whether OPM-MEG measurements of MMN responses were qualitatively comparable in terms of event-related fields, timing and topography to previous MMN findings of studies using EEG and traditional MEG. Second, we assessed the test-retest reliability (quantified by intra-class correlation coefficients, ICC) of cognitive (MMN) and sensory (auditory M100) evoked fields, comparing the sensor-level response amplitude and latency over the two separate measurement sessions.
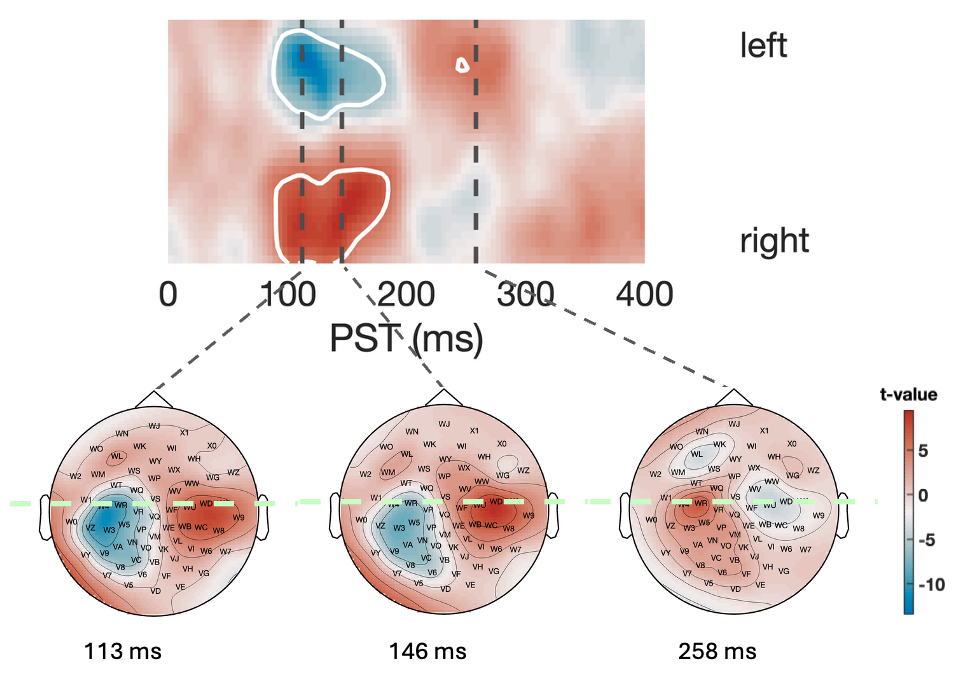
The MMN responses recorded with our OPM-MEG setup are in good agreement with previously reported MMN results of EEG and SQUID-based MEG measurements in terms of both timing and topography. For an illustration of the resulted statistical parametric map of the MMN event related radial field, see Figure 1. The qualitative comparison of group-level MMN topographies and timeseries shows excellent consistency across the two measurement sessions. Test-retest reliability analyses indicate moderate reliability for MMN amplitude (ICC=0.50-0.67 for the z-component of the triaxial sensor signal) but poor reliability for latency (ICC=0.00-0.22), in line with previous EEG literature [7]. By contrast, test-retest reliability of the purely sensory M100 auditory evoked fields has been found to be excellent for amplitude (ICC=0.97) and good-to-excellent for latency (ICC=0.84-0.96), confirming the functionality of our setup in the "out-of-the-box" state without further technical optimisation.
References
1. Boto, E. et al., Moving magnetoencephalography towards real-world applications with a wearable system. Nature 555, 657 (2018)
2. Brookes, M.J. et al., Magnetoencephalography with optically pumped magnetometers (OPM-MEG): the next generation of functional neuroimaging. Trends in Neurosciences 45, 621 (2022)
3. Holmes, N. et al., Enabling ambulatory movement in wearable magnetoencephalography with matrix coil active magnetic shielding. NeuroImage 274, 120157 (2023)
4. Näätänen, R. et al., “Primitive intelligence” in the auditory cortex. Trends in Neurosciences 24, 283 (2001)
5. Garrido, M.I., et al., The mismatch negativity: A review of underlying mechanisms. Neurophysiol. Clin. 120, 453 (2009)
6. Weber, L.A. et al., Auditory mismatch responses are differentially sensitive to changes in muscarinic acetylcholine versus dopamine receptor function. eLife 11, e74835 (2022)
7. Wang, J. et al., Test-retest reliability of duration-related and frequency-related mismatch negativity. Neurophysiol. Clin. 51, 541 (2021)