Speaker
Description
The molybdenum enzyme arsenite oxidase (Aio), is a promising biocatalyst for the detoxification and biosensor applications [1]. So far, its structural characterization and complete reaction mechanism understanding has been limited to artifactually reduced states of the Mo cofactor (Moco) caused by X-ray photoreduction (PDB 1g8k, 4aay) [2-4].
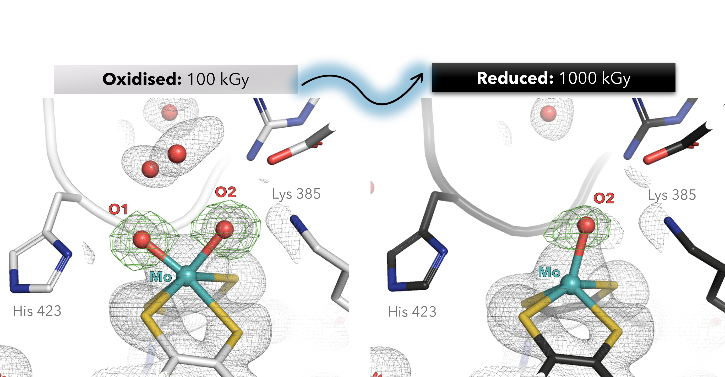
Here, we present the first crystallographic evidence of X-ray-induced photo-reduction in Alcaligenes faecalis Aio, tracking active-site geometric changes across incremental radiation doses (100–1000 kGy) at the PETRA III P14 beamline. High-resolution structures (1.5 Å) reveal that photo-reduction triggers the loss of a labile oxo ligand (O1), while displacing the Mo atom toward the dithiolene plane.
Low-dose data (100 kGy) allowed us to capture, for the first time, the Mo(VI) centre in a six-coordinated geometry with asymmetric cis-dioxo coordination (Mo–O1: 1.8 Å; Mo–O2: 2.1 Å). Reduction of the Moco also flattened the twist angle of the pterin from 31° to 17°, modulating the active site catalysis.
Our work demonstrates that radiation damage artifacts—prevalent in metalloenzyme crystallography—can obscure mechanistic insights. These findings underscore the need for dose-optimized structural data to refine AI-driven models of metalloenzyme mechanisms and advance the rational engineering of Aio for biosensing and bioremediation.
References
[1] Male, K.B. et al. (2007) Anal. Chem, 79(20), 7831–7837.
[2] Warelow T.P. et al., (2013) PLoS One, 8(8): e72535.
[3] Ellis, P.J. et al. (2001) Structure, 9(2), 125-132.
[4] Engrola, F. et al. (2023) J. Biol. Chem, 299(8), 105036.
