Speaker
Description
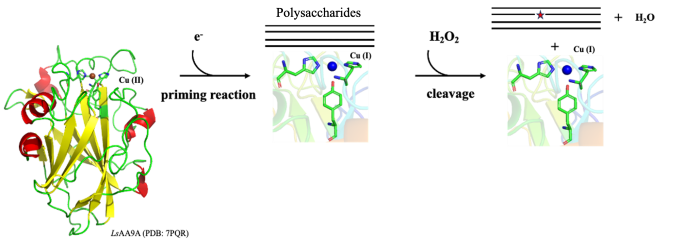
Lytic polysaccharide monooxygenases are enzymes [1] binding their active-site copper through the characteristic His-brace motif shown above including two His – one N-terminal – and often also a Tyr. The reaction cycle starts with reduction of the resting state Cu(II) to Cu(I) – in the laboratory usually using ascorbate as small molecule reductant. Despite the name, most LPMOs prefer hydrogen peroxide as co-substrate, to subsequently oxidatively cleave the glycosidic bonds in saccharides.
We have previously – through crystal cryo-structures of the model enzyme LsAA9A determined at high and low X-ray doses, and based on the hypothesis that X-ray induced photoreduction mimics natural priming reaction - reconstructed possible changes in geometry during the catalytic cycle and identified a small shortening of the Cu(II)-Tyr distance [2].
Aside from uncertainty on the biological significance of such shortening [3], we wanted to address concerns regarding the ability of macromolecular crystallography to reliably detect differences in the order of 0.1-0.2 Å. We thus carried out additional triplicate independent structure determination representing Cu(I)/Cu(II) states with/without the model substrates cellotriose, showing statistically significant differences only for the Cu(II)-Tyr distance with/without saccharide, but no other Cu-protein distance.
In order to assess whether additional general X-ray damage obscures similar shortening in the Cu(I) state induced by photoreduction, we are now comparing with cryo data collected after priming by chemical reduction with ascorbate at low X-ray doses.
Finally since X-ray-induced photoreduction of the active-site copper may closely approximate the chemical priming reaction, it holds potential as a trigger for time-resolved studies. To explore this possibility further, we are currently investigating the photoreduction process at room temperature.
References
[1] Tandrup, T., Frandsen, K.E.H., Johansen, K.S., Berrin, J.-G., Lo Leggio, L. (2018) Biochem. Soc. Trans. 46, 1431–1447
[2] Tandrup, T., Muderspach, S.J., Banerjee, S., Santoni, G., Ipsen, J.Ø., Hernández-Rollán, C., Nørholm, M.H.H., Johansen, K.S., Meilleur, F., Lo Leggio, L. (2022) IUCR Journal 9, 666-681.
[3] Wieduwilt, Lo Leggio and Hedegård (2024), Dalton Trans, 53, 5796-5807.
