Speaker
Description
Serial femtosecond X-ray crystallography (SFX) captures the structure and dynamics of biological macromolecules at high spatial and temporal resolutions. The ultrashort pulse produced by an X-ray free electron laser (XFEL) outruns' much of the radiation damage that impairs conventional crystallography. However, the rapid onset ofelectronic damage' due to ionization limits this benefit. Here, we distinguish the influence of different atomic species on the ionization of protein crystals by employing a plasma code that tracks the unbound electrons as a continuous energy distribution. The simulations show that trace quantities of heavy atoms (Z > 10) contribute a substantial proportion of global radiation damage by rapidly seeding electron ionization cascades. In a typical protein crystal, sulfur atoms and solvated salts induce a substantial fraction of light-atom ionization. In further modeling of various targets, global ionization peaks at photon energies roughly 2 keV above inner-shell absorption edges, where sub–2 keV photoelectrons ejected from these shells initiate ionization cascades that are briefer than the XFEL pulse. These results indicate that relatively small quantities of heavy elements can substantially affect global radiation damage in XFEL experiments. We go on to examine how the composition of the solvent will affect refinement.
.png)
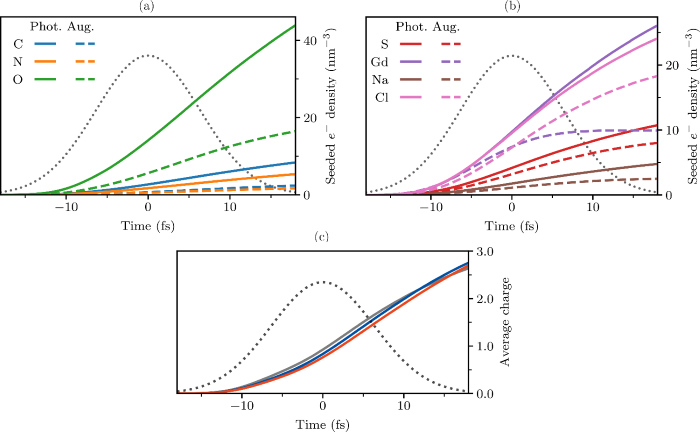
Fig. 1: Contribution of each element to secondary ionization in a lysozyme.Gd crystal (H2398C615N195O887S10Gd3Na19Cl18) under a 15 fs Gaussian pulse with a fluence of 1.75 × 1012 7.1 keV ph·µm−2. Traces show the total free electron density of the electron ionization cascades seeded by the (a) light and (b) heavy elements in the target, due to photoionization (solid) or Auger decay (dashed). Such cascades drive global ionization.
