Speaker
Description
Radiation damage is a faithful attender to X-ray crystallographic studies of metallo-proteins. Thus, care is taken to limit the effects of radiation damage and avoid consequent misinterpretation of X-ray crystallographic results. In our study, we applied defined doses to selectively probe the redox states involved in metal binding.
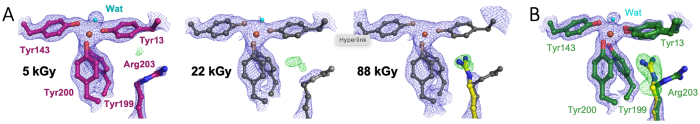
We studied the cyanobacterial iron binding protein FutA, an ABC transporter substrate binding protein that can also act as an intracellular iron binding protein [1]. We determined crystallographic structures using X-ray and neutron radiation characterised as ferrous [Fe(II)] and ferric [Fe(III)] complexes [2]. These states are distinguished by protein conformation, particularly the positioning of the positively charged Arg203 side chain as part of the iron binding site in the [Fe(II)] complex.
.png)
We captured the transition between [Fe(III)] and [Fe(II)] states upon X-ray photoreduction with a dose series using a serial synchrotron crystallography fixed target approach, see panel A. Using a novel XFEL X-ray pump-probe approach, we uncovered how Arg203 functions as a molecular switch, enabling accommodation of different iron oxidation states, see panel B [2]. The switching capability of the single FutA protein provides functional insight and suggests genome streamlining, where the loss of specialised FutA variants may reflect ecological adaptation.
References
[1] Polyviou, D. et al. (2018) J. Biol. Chem., 293, 18099-18109.
[2] Bolton, R. et al. (2024) PNAS, 121, e2308478121.
