Speaker
Description
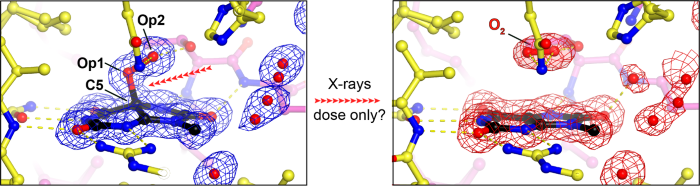
Urate oxidase (UOX) is the archetypal cofactor-independent oxidase, catalysing the O₂-mediated degradation of uric acid in many organisms. Previously, we demonstrated that the first step of this reaction is a dioxygenation reaction, forming 5-peroxyisourate (5-PIU) as an intermediate [1]. We also showed that 5-PIU is highly sensitive to radiation: during conventional single-axis X-ray diffraction experiments at 100 K, doses between 50-100 kGy caused visible damage to the C5–Op1 bond, and doses around 200 kGy led to complete bond rupture, accompanied by in situ release of O₂ [1,2]. Surprisingly, a subsequent synchrotron serial crystallography (SSX) experiment at room temperature (RT) showed no damage to this bond, even at an estimated dose of up to 70 kGy [3]. In this talk, I will discuss our more recent experiments exploring radiation-induced bond rupture under various experimental conditions—including gaussian vs. top-hat beam profiles, cryogenic vs. room temperature, and serial vs. conventional crystallography—in an effort to better connect these observations to the reaction mechanism.
.png)
References
[1] Bui S, von Stetten D, Jambrina PG, Prangé T, Colloc'h N, de Sanctis D, Royant A, Rosta E, Steiner RA (2014), Angew Chem Int Ed, 53, 13710-13714.
[2] Bui S and Steiner RA (2017) Curr Opin Struct Biol, 41,109-118.
[3] Zielinski KA, Prester A, Andaleeb H, Bui S, Yefanov O, Catapano L, Henkel A, Wiedorn MO, Lorbeer O, Crosas E, Meyer J, Mariani V, Domaracky M, White TA, Fleckenstein H, Sarrou I, Werner N, Betzel C, Rohde H, Aepfelbacher M, Chapman HN, Perbandt M, Steiner RA, Oberthuer D (2022) IUCrJ, 31:778-791.
